Bringing a complete portfolio of design, prototyping, simulation, testing, and development under one roof, our fully merged development cycle enables us to offer an optimized design control package in line with FDA policies for medical device design and development for components and assemblies.
Our design and development engineers boost your idea from concept to completion, including, in the process, the most sophisticated methods and technologies in the medical device industry. At Seisa, we diligently conduct reviews, hazard identification, and risk analysis throughout our documented process.
Why is Medical Device Design Important?
The design and development of a medical device is the most vital phase for its success. A loosely defined and designed medical device cannot concede with the regulatory needs and make it to market.
Even if it passes the compliance, it will fail to deliver the limited functionality and benefits, according to the market conditions, and suffer from lesser market adoption reached to well-designed products.
At Seisa Medical, we are Expanding hours with medical device developers and analyzing multiple Medtech projects, including an allergic drug vending machine, endoscopy device Miniaturization, software-controlled insulin pump, and many more related medical types of equipment, now medical device design and development is more than just conceptualizing a solution, developing a prototype and mass manufacturing to sell.

What is the process of designing a medical device?
Solidify your Medical Device Design, Development, and Manufacturing from the beginning.
Design For Manufacturing guide, Supply Chain focus, Regulatory Approval, Volume Scale Preparedness
One of SEISA’s best benefits lies in our vertical integration strategy that contains concept to Commercial approval, scale production, and maintenance to prevent Supply Chain risk. We have experience with a wide variety of medical devices, and our experts have high expertise in medical device design and manufacturing. This allows SEISA to “marry” design and manufacturing for better results.
Medical Device Design Control Process
The initial phase from which Design Control begins is Design Input development and approval, which consists of device design and manufacturing processes to be moved out into the production phase.
Design control is a holistic strategy and doesn’t end with sharing the design to the production phase once the design is finalized. It also moves manufacturing processes according to the changes in the design stage or even post-production feedback. It is a continuous process to develop a usable product for a user and, for the improved product, consider revolutionary changes from usage patterns and diagnose failed products.
The image below shows how Design Control can be performed in the waterfall design process.
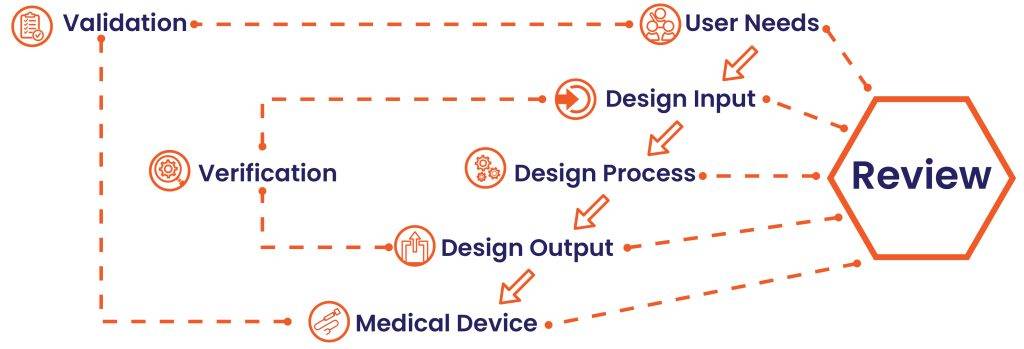
Step 1: User Needs
Requirements are specified considering the market need, and the device is designed to manage that need. After a sequence of evolution, the medical device design is concluded and transferred to production for manufacturing. There is a need for feedback during every stage of this process.
Step 2: Design Input
This is an iterative process. When an organization fixes to address a particular need, they review and test the acceptability of design input derived from the market. At that point, the iterative process of altering requirements into device design forms.
Step 3: Design Process
These design inputs are transformed into design outputs by converting those needs into high-level specifications.
Step 4: Design Output
The verification process ensures whether the specifications satisfy requirements. And the output becomes the input to modify the criteria, and this method continues until Design Output is aligned with the Design Input.
Step 5: Medical Device
Once the final design is ready, it is shared with the production facility for mass manufacturing.
Testing – Verification and Validation
Every medical device must fulfill the functionality, usability, and reliability objectives to get a successful share of the market. Apart from these, end users also look for the effectiveness and safety of devices they use to manage a particular problem or situation, which are sometimes critical to life. This is why iterative testing with verifying and validating these medical devices becomes compulsory.
Medical devices may consist of various technology shapes, sizes, and different levels of complexity. Verification and validation (V&V) action is guided by the regulatory environment and must follow international standards.
Our Standardized V&V activities can simplify the manufacturing process and improve the approval process. Additionally, automatic testing, diagnostic techniques, and data collection tools can enhance the V&V procedure.
The complexity of any testing process depends on the technologies used and the geographical target markets. The test strategy should obscure at least six parameters mentioned below:
- Targeted geographies and associated standards
- Time to market demand
- A measure to be observed with the version
- Testing Labs – internal or external labs
- Defining the arrangement of tests
- Presenting the test result
Design Control Regulations
Medical device manufacturers need to obey Design Control guidelines since regulatory bodies like FDA, European Commission, Health Canada, and others want to ensure that the medical devices are safe for possible users before manufacturers start to market the instruments.
The FDA doesn’t follow ISO 13485 as it has different conditions for quality management. Design controls are defined under FDA 21 CFR 820.30, which has an identical intent to section 7.3 Design and Development described under the guidelines for ISO 13485.
Additionally, FDA incorporates Current Good Manufacturing Practice (CGMP) requirements into the quality system regulation to follow good quality practices for medical device design.
To successfully execute design control of medical devices, Siesa meets professionals with both technical and non-technical backgrounds, such as business management, life science, engineering, computer science, and the arts are required.
Design & Development Services Benefits
Seisa is an extension of your development team and can support you from the start of your project. We partner with our customers by supplying premier engineering from start to finish, developing value-engineered products. We have all the essential resources to integrate our activities into our client’s product development and supply chain methods so our clients can realize substantial benefits.
IP Protection:
Intellectual property safety for medical products. Aside from Non-exposure deals, SEISA maintains robust systems to protect customer data. Access only for the design department with a separate server system.
Iteration development:
- Dedicated equipment for fast extrusions and injection molding. Fixtures and assembly equipment are ready for iterations.
- A team of program managers and engineers is ready to help with your medical device design and iteration demands.
- Dedicated Bench Testing Equipment.
- SEISA’s design and development lab can perform most bench tests as required by MDR, ISO 13485, and guidance by FDA for 510k approval.
Reduce Manufacturing Costs
Our capabilities cover a broad range of medical device products. Our team members have extensive experience in the design, development, and manufacturing of medical devices, giving us a first-hand experience with the industry’s product development needs. We’re well-versed in the latest technology, including usability, software, and equipment.
With deep experience in both the front-end design and back-end manufacturing phases, SEISA’S team can design high-performing medical devices that can also be efficiently and cost-effectively manufactured. Our design for manufacturability expertise can reduce the complexity, reducing both cost and quality issues while assuring long-term, efficient manufacturing operations.
As an extension of your business’s R&D department, we rapidly develop a detailed plan that minimizes steps but optimizes material yield.
Accelerate Time to Market and Minimize Risk
Our concept-to-supply business model accelerates time-to-market and reduces risks for our clients. We can get you there with ISO and QSR compliance, state-of-the-art skills, competitive costs, and an established network of global connections, including the Far East.
We always have your finish line in mind. We respond promptly to your demands and can adjust planning and undertaking rapidly to adapt to any changes that may arise. Our knowledge of the regulatory needs and the entire commercialization process affords our clients the trust that their products will launch on time.
Strengthen Intellectual Property and Gain Technology Expertise
We can help you strengthen your intellectual property portfolio or create a matrix of existing art to specify opportunities and points of weakness. Our industry experience provides a thorough review, and we can offer distribution of your company’s products across all medical device markets.
In addition, we provide
- Design control.
- Compliance-related documentation tracking
- Regulatory filings.
- Domestic and offshore sourcing of elements and subassemblies.
Medical Device Design and Development Case Study
- One challenge in our industry is companies outsourcing the full development of their medical devices. Likewise, start-ups often piece together a risky piecemeal supply chain from the design start.
- Smaller companies must refrain from taking on FDA or MDR registration for complicated and challenging supply chains.
- Companies may choose critical medical device components from companies needing more financial or operational feasibility to supply parts competitively in the long term. This can hurt a company’s ability to procure in the future or affect its valuation when selling.
- SEISA helps customers streamline their supply chain from the start.
- Select from an array of in-house services
- Design your supply chain from scratch with SEISA’s team of experienced and qualified engineers.
- From the beginning, streamlining Design For Manufacturing and Supply Chain avoids short- and long-term issues.
Product Design & Development FAQ
Q. What is the Medical Device Development Process?
A. The medical device development process is a set of practices and procedures with specific phases to ensure good quality practices so that the medical device design is effective and safe for its intended use.
As a result, the medical device development process covers the entire life cycle of a medical device, from the concept to its commercialization.
Q.How are medical devices designed?
A.Some general steps in the medical device product development life cycle are followed across the board for the medical device design. These steps include the concept or initiation, formulation, design and development, design verification and validation, final validation, product launch, and postlaunch assessment.
Q.What are essential design considerations for medical devices?
A.The essential design considerations for a medical device include the performance of the device, safety features or characteristics, sterilization, materials selected and their biocompatibility, usability engineering or human factors, compatibility with other medical devices, labeling, packaging, shelf-life, and regulatory requirements.
Q.What should you look for when selecting a medical device design company?
A.It would help if you were looking for a medical device design company with a Quality Management System where the ability to provide medical device design and related services that consistently meet customer and applicable regulatory requirements is demonstrated. As well as the capability of creating the concept, product design, prototyping, testing for verification and validation, manufacturing, and experience to make the commercial submission to a regulatory body, such as the Food and Drug Administration (FDA) in the United States.
