
IQ, OQ, PQ validation: What do they mean?
Learn about the medical devices industry’s IQ, OQ, and PQ validation processes. These processes are crucial for ensuring the safety and efficacy of products and complying with industry regulations.

Learn about the medical devices industry’s IQ, OQ, and PQ validation processes. These processes are crucial for ensuring the safety and efficacy of products and complying with industry regulations.

Discover how a proactive approach to quality improvement, including design for manufacturing principles and process control, can increase product consistency and reliability while reducing scrap and rework.
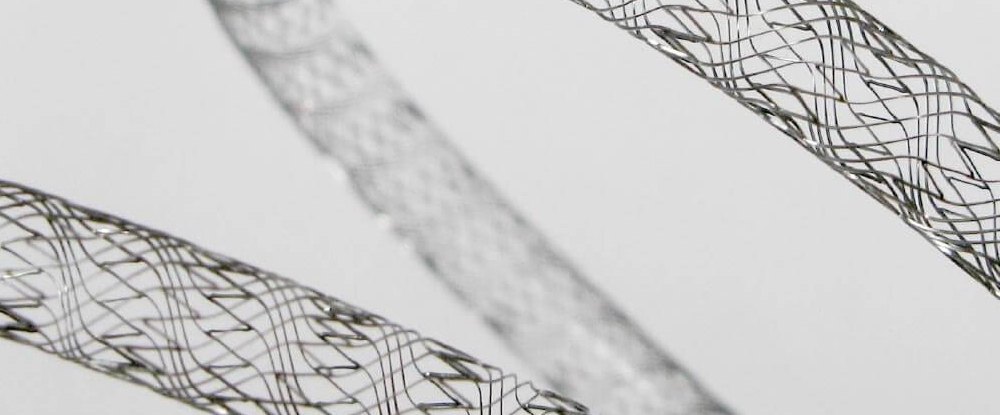
Nitinol wire is a shape memory alloy (SMA) with unique properties and a wide range of applications in medical and industrial fields. In this blog
The classification of medical devices proposed by the FDA is almost a worldwide standard. Find out what is Class II and Class III.

Learn about our global vision and how we have impacted the medical device industry by expanding into Mexico, the United States & Europe.