Medical Device Injection Molding
Seisa’s medical device molding process allows you to leverage the speed of development you are used to in the highly regulated environment of the medical space. Our team of medical molding manufacturing experts ensures Class II and III devices quickly from prototype to production.
Lead times can be completed as quickly as five days to create your prototypes so that you can manage potential compliance issues early on and get your product to market faster. Both prototyping and medical device injection molding are manufactured at our ISO-certified locations.
Manufacturing medical devices require a high degree of accuracy, and a single micron or millimeter can determine whether a part meets specifications. SEISA follows and performs all the fundamental guidelines to produce volumes of devices and components while ensuring minimal to no variance between the pieces.
Injection Molding in the Medical Field
The primary conditions for injection molding of medical plastics are biosafety and chemical strength, since these factors will connect with the human body in one form or another. It is paramount that these materials must not cause detriment to the human body or leave harmful substances.
Medical injection molding is used for numerous medical products, including the following:
- Beakers, containers, and test tubes
- Housing and casings for lab and medical equipment
- Implantable components
- Drug delivery components and equipment
- Orthopedics
- Surgical components and equipment
Materials Used in Medical Molding Applications
Different materials are usually used in injection molding for medical devices and supplies. Plastics have been used over other materials since they offer cost-savings, design flexibility, and rapid production. Some materials used for medical injection molding include:
Polycarbonate
This is a strong material with extensive vibration and influence resistance. It can be used for medical components, especially when visibility is a priority. This material is also resistant to UV light and heat.
Polyethylene
This material has a high molecular weight and performs well for prosthetics and other devices that can be modeled based on its durometer, impression, and increased durability.
Polypropylene
This material is beneficial for components that require multiple utilizing of an autoclave since this material has high heat resistance characteristics. This material is also resistant to radiation
Silicone
When flexibility is a design requirement, the use of silicone for components is a recommended option. It has excellent biocompatibility and durability characteristics and is also inexpensive, mainly when producing high volumes.
Polyetheretherketone (PEEK)
Is a thermoplastic material known for its high performance and excellent mechanical properties. It has a high-grade resistance to radiation and thermal degradation.
We also use other materials that are common and highly accepted by the medical device industry:
- ABS
- Polystyrene
- COC
- Acrylic
- TPEs and TPUs
CNC Machining and EDM Capabilities For plastic mold design
Our expert engineers work in our industry-leading facilities and work on various materials and processes. Seisa’s Metal Machining Center of Excellence is located in the San Francisco Bay Area in Northern California and is responsible for fabricating and delivering various products, including mission-critical medical device components and assemblies.
Machining and Fabrication Capabilities
- CNC Mill, Lathe
- Mill-Turn
- Metal Stamping
- Wire Forming
- Needle Sharpening and Forming
- Wire EDM (down to .004″ wire)
- EDM Fine-Hole Drilling (down to .002″ diameter)
- 3D Printing
Types of Materials Machined
- Stainless steel
- Aluminum
- Brass
- Copper
- Titanium
- Magnesium
- Other exotic metals.
Processes
- CNC Programs: SurfCAM, GibbsCAM, Fusion 360, Esprit
- Design: SolidWorks, AutoCAD
- Mold Design
- Mold Flow Analysis
- Design For Manufacture
Why partner with Seisa Medical?
When making mold projects, we are mindful of choosing the type of mold steel and prefer the most optimal mold steel grade for our clients. In Seisa’s production and machining areas, we employ high-quality materials from Europe and Asia for the molds we produce.
Before commencing a mold project, we ensure the following are discussed:
- Gating path will be designed.
- Mold surfaces will be examined.
- Mold cooling channel design will be scanned.
- Operation process flow will be reviewed.
- Type and size of molding machine will be evaluated.
- Mold design (i.e. inserts, MUD, etc.) will be established.
Our Injection Molding Capabilities
When it comes to plastic injection molding capabilities, if it fits in our mold, we can make it. Since 1983, we’ve maintained the highest quality equipment and maintenance standards to consistently deliver quality-to-price solid ratios. We inspect and test 100% of our mold to ensure the best quality.
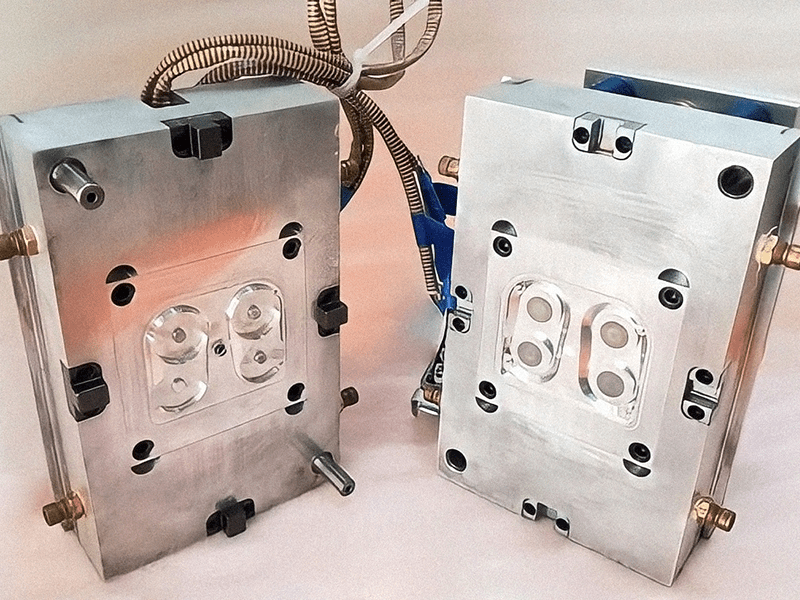
Mold Making
Our competence and abilities are centered around combining digital manufacturing technologies with conventional manufacturing processes. This is one advantage of how we are able to rapidly employ high-quality 3D designs to manufacture high-accurate mold tools in a matter of just a few days.
We offer rapid tool fabrication, mold making, and die manufacturing for prototyping as well as low-volume and mass production. Our professionals design and utilize steel and aluminum tooling. Additionally, our staff conducts regular mold maintenance to ensure the optimal performance of the tools.
Plastic Injection Molding
We utilize high-quality thermoplastic materials to produce top-grade plastic injection molded parts as quickly as seven days. From our fast turn-around quoting service to design evaluation, DFM, toolmaking, die, mold production, and ultimately injection molding, we are committed to always maintaining Quality. Furthermore, our injection molding capabilities include insert molding and over-molding.
Micro injection molding
Micro injection molding requires the highest care to ensure the outcome of products will meet specifications. Our state-of-the-art manufacturing equipment are readily available for micro molding operations.
Controlled Environment Rooms for Medical
Most of the injection molded medical devices that we manufacture are produced in ISO Class 8 (Class 100,000) CER – Controlled Environment Room. In compliance with our Quality Management System that is established by our ISO 13485:2016 Certification, the clean rooms are maintained accordingly and demonstrate excellent contamination-free conditions. Our cleanrooms are critical components in our Operations for ensuring traceability, lot-to-lot tracking, establishing an aseptic workflow, and for monitoring air quality to identify and eliminate sources of contamination.
Medical Plastic Injection Molding Benefits
Since medical device plastics deliver increased versatility and can be mixed with metal to create enhanced medical product qualities, medical-grade plastics have become the chosen material for multiple electronic medical devices. Some of the benefits that SEISA’s molding Plant provides:
- Improved ergonomics
- Reduced weight
- Increased functionality
- Lower cost
- The decreased burden of sterilization