Product Design & Development
We have a team of experts with extensive experience in medical device design. We follow a fully integrated development cycle to provide optimized design control packages that meet both FDA policies and regulatory requirements.
We understand that the success of a medical device relies heavily on its design and development phase. Therefore, our team works diligently to ensure our products meet regulatory needs and market demands.
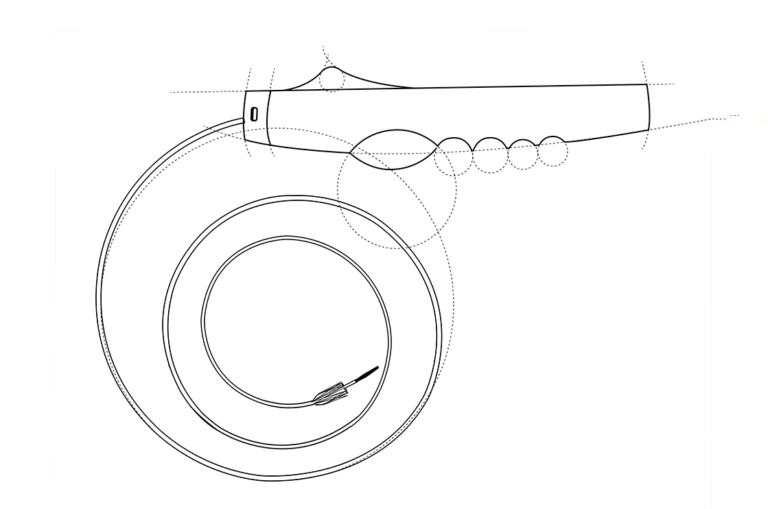
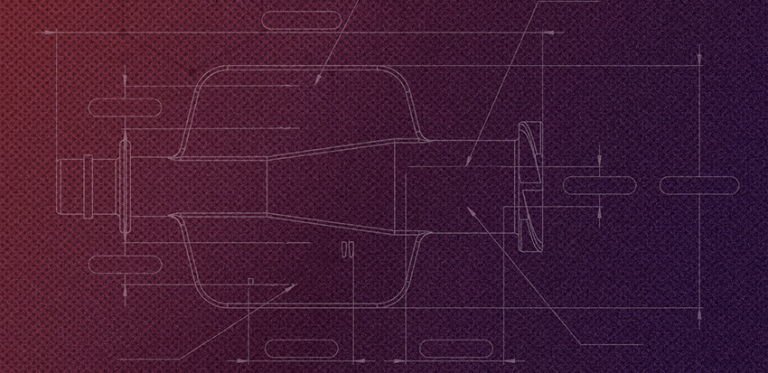
We use the latest methods and technologies in the industry to bring your ideas to life and take them from concept to completion.
Our vertical integration strategy allows us to handle everything from design to commercial approval, scale production, and maintenance. This approach reduces supply chain risks and improves efficiency.