IQ, OQ, PQ validation: What do they mean?

Learn about the medical devices industry’s IQ, OQ, and PQ validation processes. These processes are crucial for ensuring the safety and efficacy of products and complying with industry regulations.
Most common defects on Injection Molding: How to improve quality

Discover how a proactive approach to quality improvement, including design for manufacturing principles and process control, can increase product consistency and reliability while reducing scrap and rework.
Nitinol: Properties, Applications, and Uses
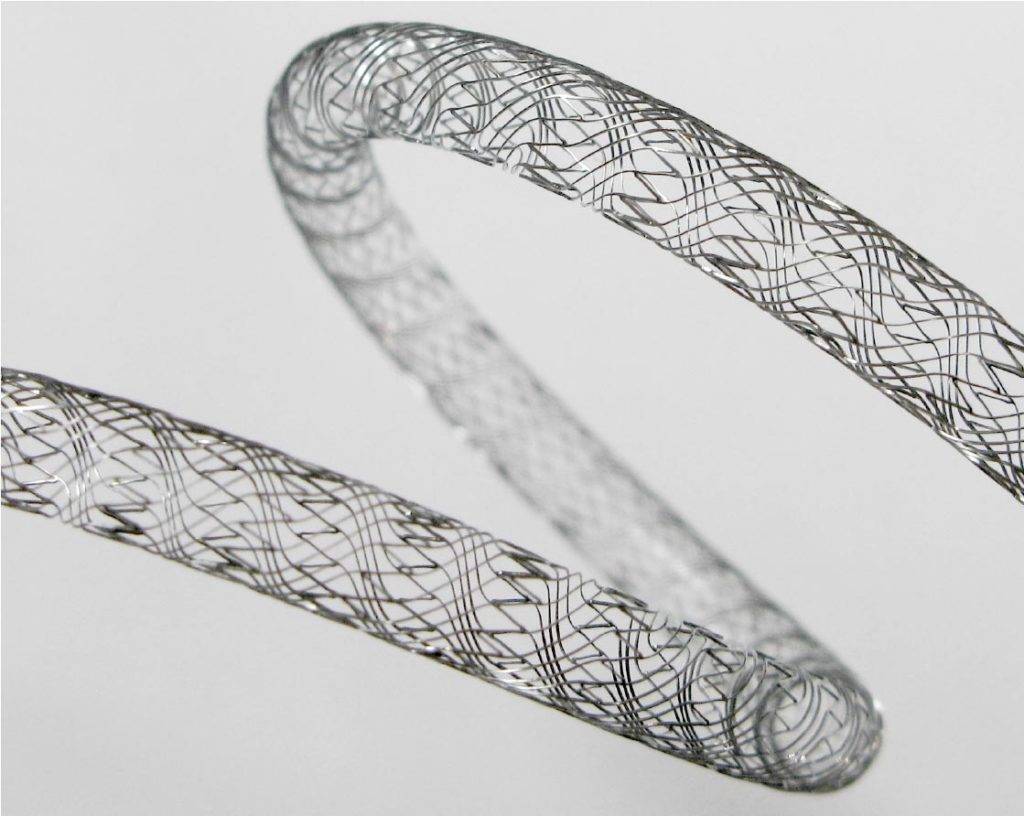
Nitinol wire is a shape memory alloy (SMA) with unique properties and a wide range of applications in medical and industrial fields. In this blog post, we will explore the properties and uses of Nitinol wire, how it is made and what the future holds for this fantastic material. Introduction to Nitinol: Properties and Applications […]
What determines a class II & III medical device?
The classification of medical devices proposed by the FDA is almost a worldwide standard. Find out what is Class II and Class III.
SEISA’s global presence

Learn about our global vision and how we have impacted the medical device industry by expanding into Mexico, the United States & Europe.